Experience Chemistry
EXPERIENCE CHEMISTRY is a series of innovative apps designed to motivate students to investigate basic scientific phenomena and concepts in chemistry through simulations and interactive labs. Using an inquiry-based learning approach, the apps challenge students with investigations and quizzes based on the students explorations of each interactive unit.
EXPERIENCE CHEMISTRY PROVIDES APPS WITH:
• Dynamic simulations of scientific phenomena and concepts, such as states of matter, floating and density, dissolving sugar or salt in water, diffusion rate, the Law of Conservation of Mass, and the structure of the atom;
• Multiple representations, such as dynamic graphs, tables, and interactive animations;
• Investigations that help students learn by exploring inquiry questions;
• Thought-provoking quizzes that are ideal for individual learning, small-group work, and teacher-led class instruction;
• Scientific background of the relevant concepts introduced in each unit.
EXPERIENCE CHEMISTRY UNITS ARE:
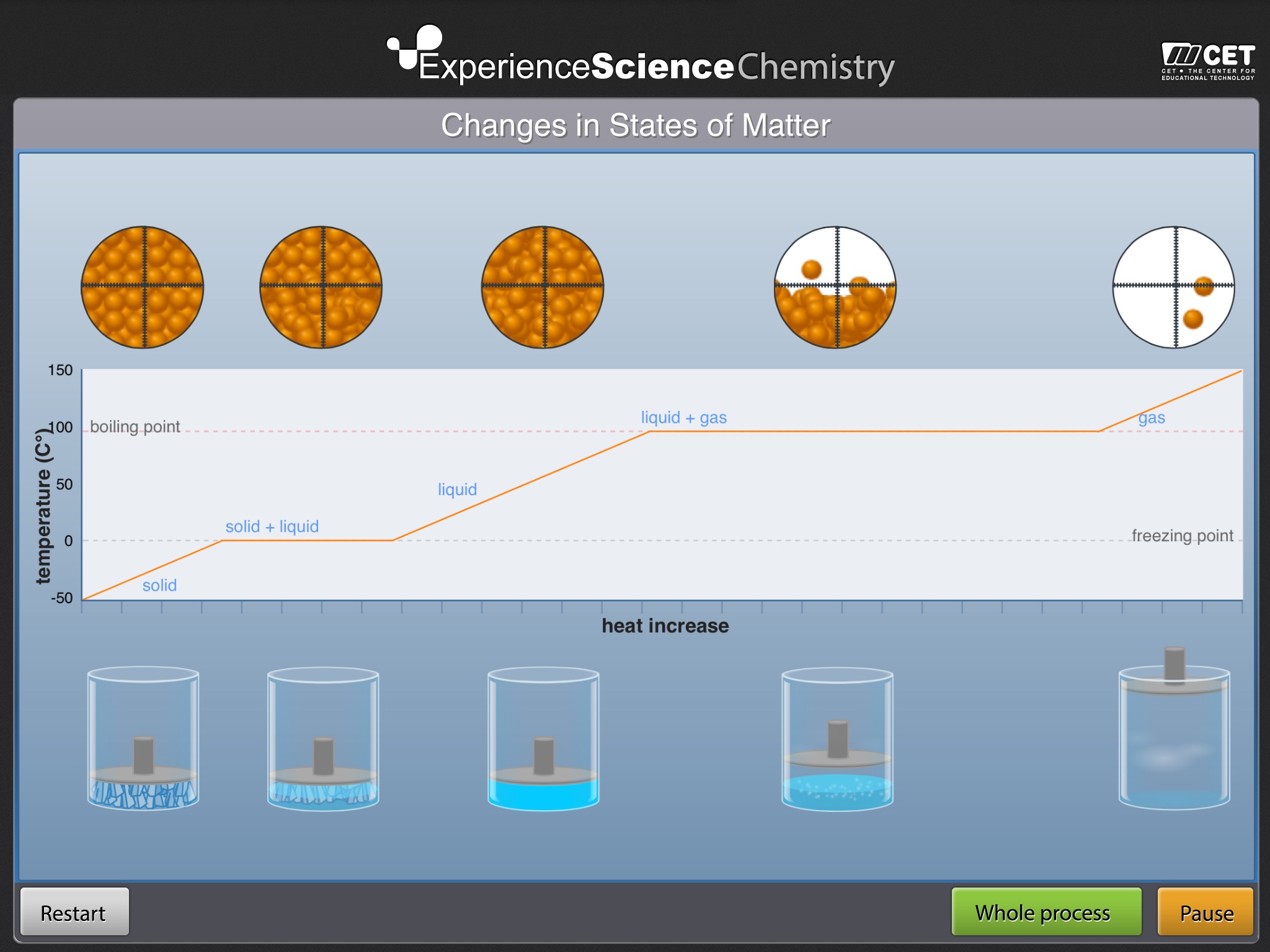
CHANGES IN THE STATE OF MATTER invites students to observe the various changes that occur when water is heated and transitions from ice to liquid to gas.
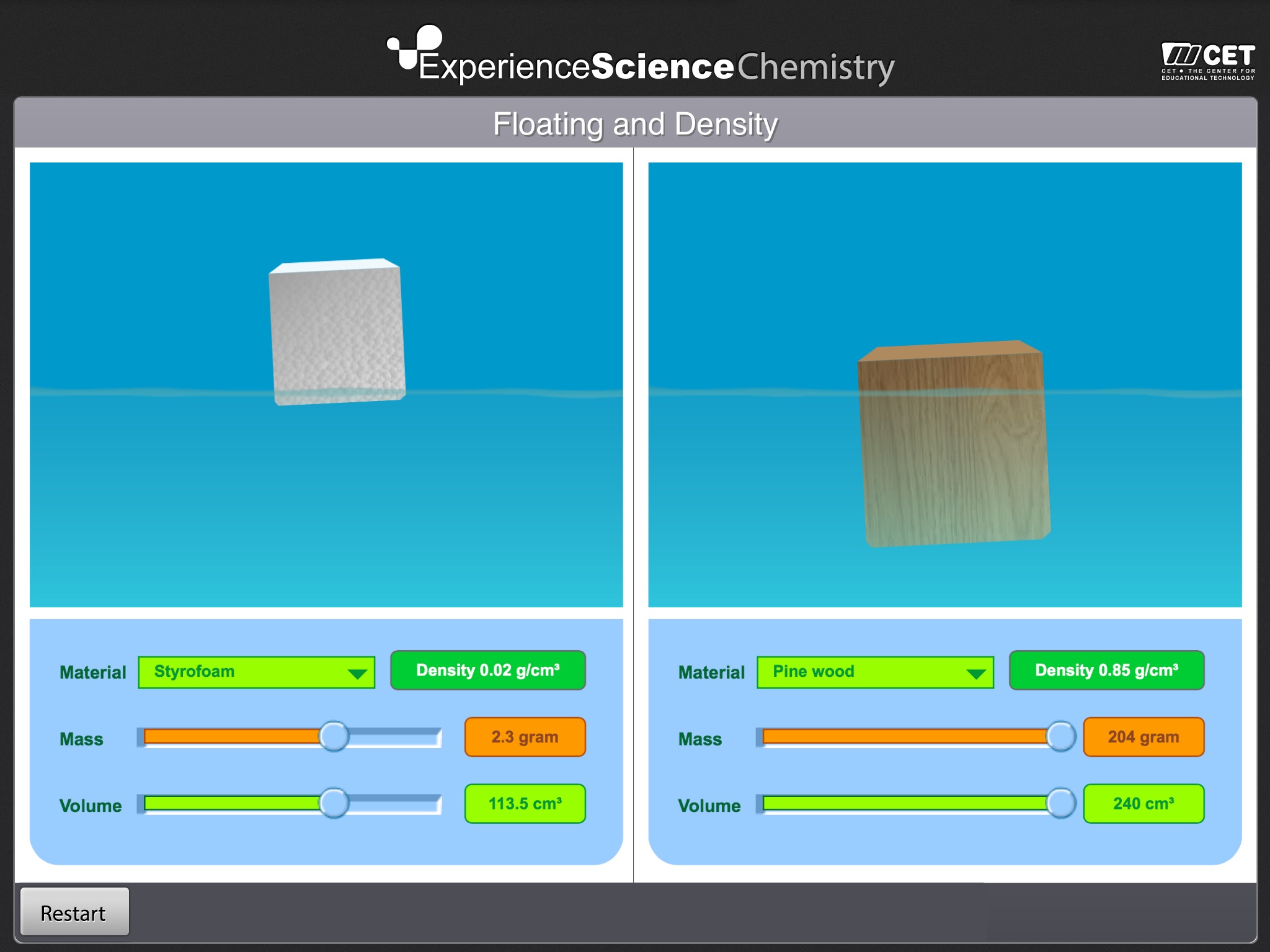
Floating and Density invites students to place cubes of different materials in water tanks and understand what causes them to float or sink.
DISSOLUTION invites students to observe a microscopic view of how salt and sugar dissolve in water.
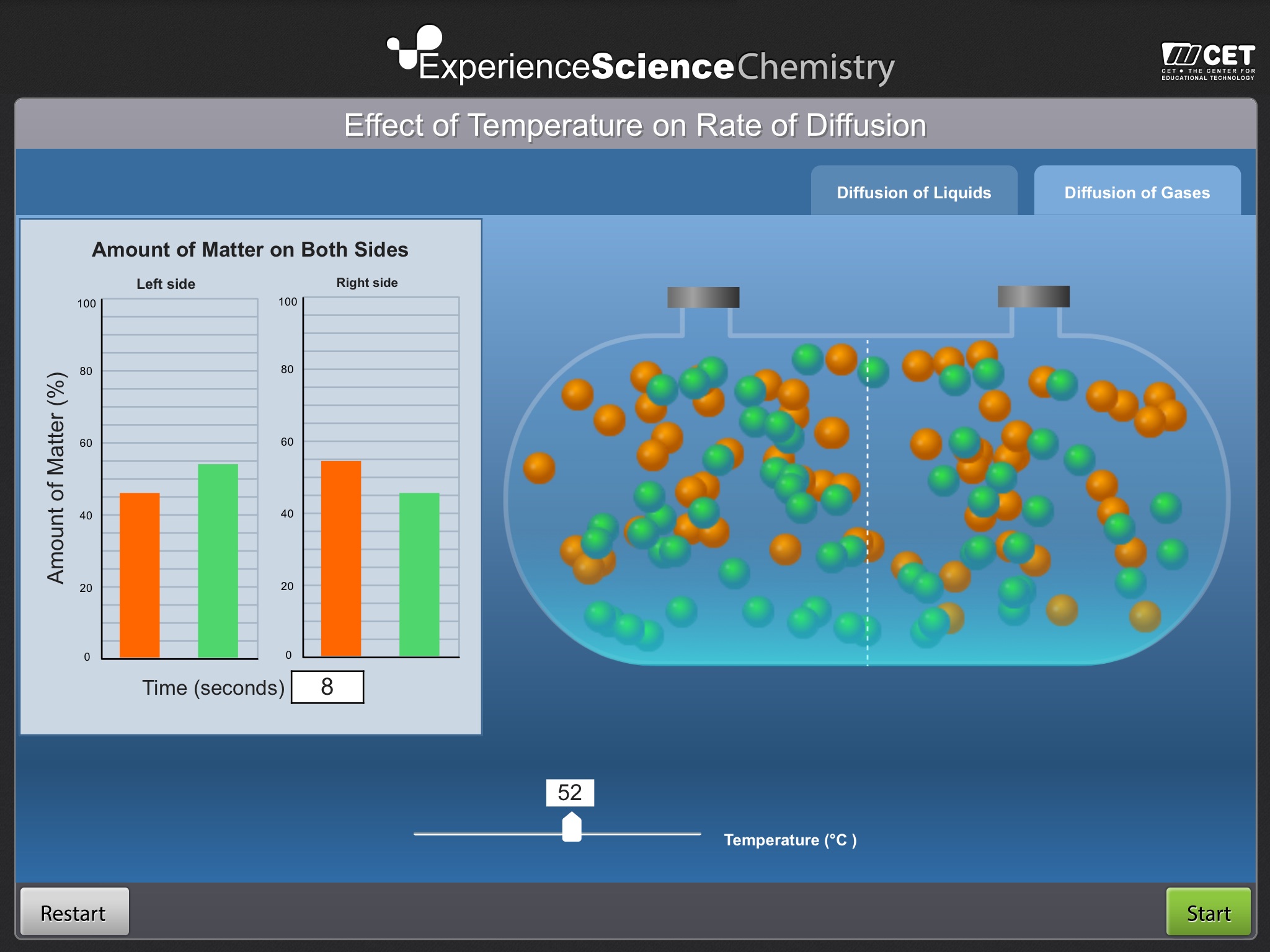
Effect of Temperature on Diffusion Rate invites students to remove the barrier between two parts of a vessel, each containing a different substance, and measure the resulting diffusion rate at different temperatures.
THE LAW OF CONSERVATION OF MASS invites students to compare the weights of the reactants and products of different chemical reactions in order to test the Law of Conservation of Matter. The reactions can be conducted in open or closed vessels.
The Structure of the Atom invites students to construct specific atoms and ions in a partial Periodic Table of Elements by choosing the correct number of protons and electrons for each.